Abstract
Background:
IGH and TCR gamma clonality testing is an important component in the diagnosis of lymphoproliferative disorders. Capillary electrophoresis (CE) of multiplexed PCR products is currently considered the gold standard method of analysis. Although robust, simple and highly reproducible, it does not provide full characterization of clonal sequences and lacks sensitivity and specificity to track clones in subsequent samples. Here we describe our clinical implementation of an NGS based assay for routine assessment of lymphoproliferative disorders and outline the clinical utility for initial clonal characterization and minimal residual disease monitoring through patient specific clone tracking.
Methods:
Blood, bone marrow and FFPE tissue samples submitted for routine clonality assessment during Jan 2016 to March 2017 were identified. DNA was extracted and tested using both standard CE and LymphoTrack®IGH + TRG - MiSeq assays (InvivoScribe). Results were analyzed using an in-house developed software (LymphoClone) as well as proprietary InvivoScribe software. The lymphoma classification at the time of initial diagnosis, disease status and results of ancillary studies, including morphologic assessment, immunohistochemical analysis, flow cytometry (FC) and molecular test results were recorded for each case.
Results:
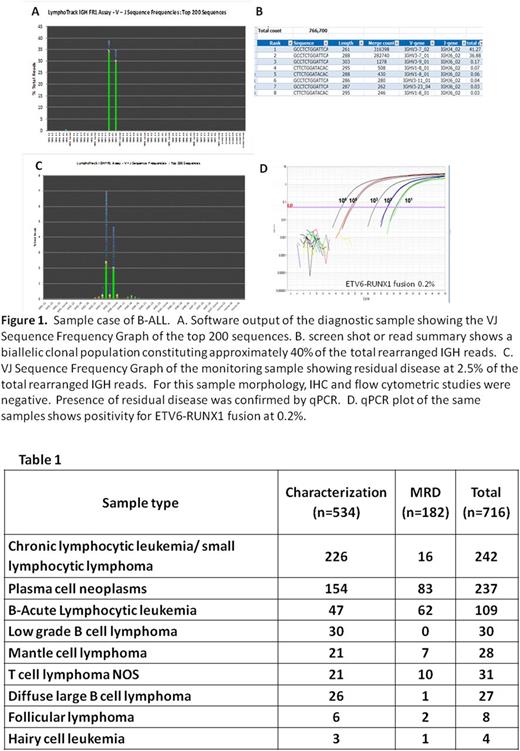
A total of 716 samples were analyzed, including 534 samples at the time of initial diagnosis (Dx) for clonal characterization and 182 follow up samples for disease monitoring. The case distribution is outlined in table 1. Sensitivity for initial clonal characterization was superior for the NGS assays, allowing clonal detection down to 1% tumor content, compared to 5-10% for the CE assays. Clonal rearrangement was detected in 94% of Dx cases by NGS (502/534) vs 89% CE (475/534). For monitoring samples with a previously characterized clone, NGS was equivalent to FC for detection of plasma cell neoplasms (PCN) but showed distinct advantages in disease monitoring for B-ALL, B and T cell lymphomas. Among 182 monitoring samples, NGS testing allowed residual disease detection in 25 (14%) more cases (137/182 vs 112/182) compared to flow cytometry. In all cases, residual disease was confirmed by an ancillary method (qPCR for a molecular marker) or a subsequent sample with higher disease level. The sensitivity for MRD detection for NGS has been established as 1x10-5. Sensitivity for FC ranged between 1x10-4 to 1x10-5 (1x10-4 for T and B-ALL and 1x10-5 for PCN and B cell lymphomas). Higher sensitivities may be attained by NGS with increasing DNA input. The NGS assay provided additional information on distinct clonal tumor population behavior, including suppression and/or re-emergence of subpopulations following treatment. In the current study, turnaround time (TAT) was higher for NGS vs FC, 9 days vs 1 day, respectively.
Conclusions:
Routine assessment of clonality by NGS methods is feasible and provides significant improvement over existing CE clonality assays, enabling comprehensive clonal characterization and disease monitoring with high sensitivity which is similar and often superior than FC. Based on current technology and protocols, TAT is longer for NGS compared to both CE and FC assays, which could limit its use as a stand-alone test in select clinical settings. Further protocol optimization is in progress to reduce this time to ~7 days. The NGS assays further define behaviors of clonal tumor populations and should therefore be considered in the routine assessment of lymphoid malignancies in conjunction with FC and other ancillary molecular tests.
Arcila: Raindance Tecnologies: Honoraria; Invivoscribe: Honoraria; Archer: Honoraria. Park: Amgen: Consultancy. Dogan: Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Peer Review Institute: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche Pharmaceuticals: Consultancy; Celgene: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal